Heat pump
Take 15 minutes to prepare this exercise.
Then, if you lack ideas to begin, look at the given clue and start searching for the solution.
A detailed solution is then proposed to you.
If you have more questions, feel free to ask them on the forum.
It has two pools of masses
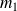 and
and
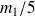 .
.
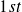 is desired to convert into heated pool and
is desired to convert into heated pool and
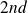 into an ice rink with a heat pump operating reversibly.
into an ice rink with a heat pump operating reversibly.
The specific heat capacity of water
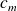 is given.
is given.
Question
Initially,
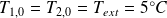 .
.
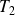 decreases by
decreases by
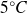 .
.
Determine the final temperature
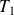 and the work
and the work
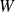 to provide.
to provide.
(Hint : consider a low temperature variation on an elementary cycle).
On an elementary cycle :
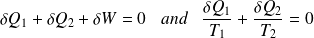
Yet :
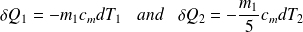
So :
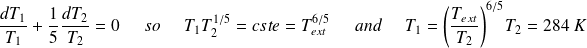
The provided work is :
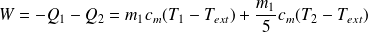
Question
In a
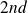 stage, the second water basin passes its frozen state.
stage, the second water basin passes its frozen state.
The specific latent heat of water is
 .
.
Determine the new final values of
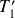 and
and
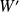 .
.
Then :
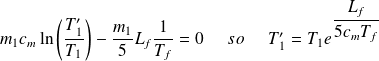
Work is :
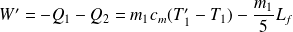
Question
In a third step, the temperature of the ice is lowered by
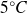 .
.
Determine the new final values
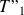 and
and
 .
.
Reasoning identical to that of the question
 .
.





