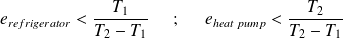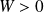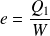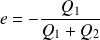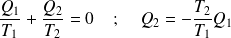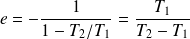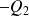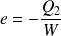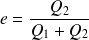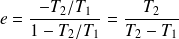The thermal machines
Fondamental : Beau de Rochas theoretical cycle (1862) - the 4 stroke internal combustion engine
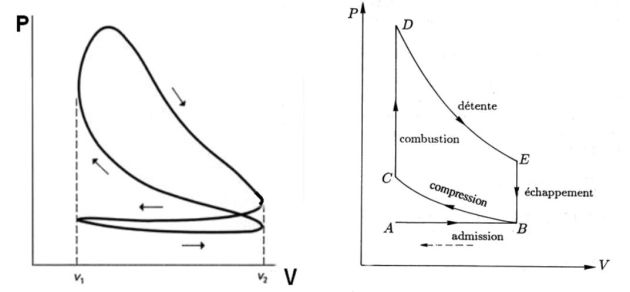
The following figure shows the
 stroke internal combustion engine.
stroke internal combustion engine.
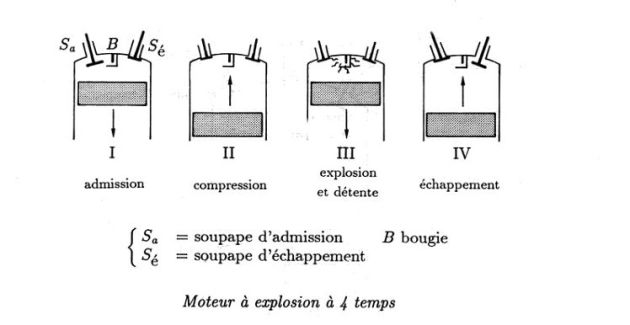
The experimental diagrams (left) and theoretical (right) are available in the following figure.
A video that shows the internal combustion engine :
Simulation :
The steam engine seen by "The Shadoks" (Référence : aaa production)
"The drinking bird", an example of a thermal machine ?
Fondamental : Study of Beau de Rochas cycle - Efficiency calculation
Simplifying assumptions :
During the cycle, the fluid properties change. We do not take it into account and considering the gas as a perfect gas.
This is always the same gas that undergoes the cycle.
The transformations are reversible :
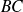 : adiabatic compression
: adiabatic compression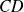 : isochoric (spark from the spark plug)
: isochoric (spark from the spark plug)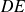 : adiabatic expansion
: adiabatic expansion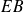 : isochoric cooling
: isochoric cooling
Cycle efficiency :
The cycle is motor (
 ) : the fluid during transformation
) : the fluid during transformation
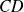 receives heat and gives heat to the external medium during
receives heat and gives heat to the external medium during
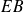 transformation.
transformation.
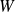 means the sum of the works received by the gas during the cycle (that is to say during the
means the sum of the works received by the gas during the cycle (that is to say during the
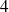 transformations).
transformations).
Cycle efficiency (of the motor)
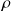 is defined by :
is defined by :
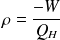
Where :
 is the work done by the system (energy exiting the system as work).
is the work done by the system (energy exiting the system as work).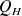 is the heat put into the system (heat energy entering the system).
is the heat put into the system (heat energy entering the system).
Either here :
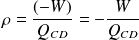
According to the first law :
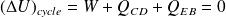
Is :
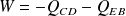
The efficiency expression becomes :

Yet (isochoric transformations) :
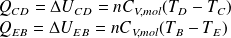
Whence :
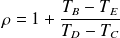
For both isentropic (reversible adiabatic), we can write :

And noting that
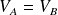 and
and
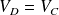 , we obtain :
, we obtain :
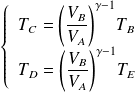
We deduce the expression of the efficiency :
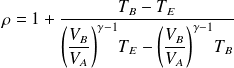
Or, finally :
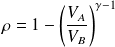
We note
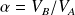 the compression ratio :
the compression ratio :
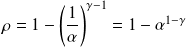
For
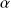 between
between
 and
and
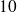 and with
and with
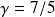 ,
,
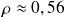 .
.
Fondamental : The Carnot heat engine
General principle of a heat engine :
The figure below shows the principle of a Carnot engine (dithermal machine).
A fluid undergoes transformations of cycles in which it exchanges work and heat with the outside.
If the fluid "effectively" provides work to the outside, the machine is a motor.
If the fluid receives work and takes heat from the cold source, the thermal machine is a refrigerator (or air conditioner).
If the fluid receives work and provides heat to the hot source, the thermal machine is a heat pump.
If the fluid exchanges heat with two heat sources, the machine is dithermal.

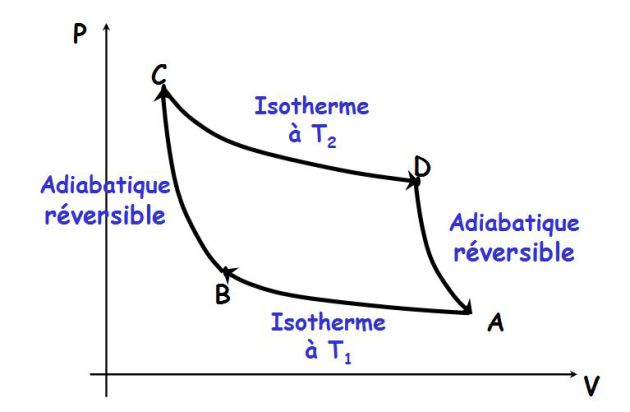
The cycle of reversible Carnot engine (dithermal motor cycle) :
The block diagram is shown in the following figure.
The fluid receives heat from the heat source, provides work to the external environment and rejects a portion of the heat energy received from the cold source (impossibility of monothermal engine).

The cycle consists of two reversible adiabatic (no heat exchange) and two isotherms (in contact with two heat sources).
It is drawn into the Clapeyron plan in the following figure.
Yield (motor efficiency) calculation :
The yield is defined as :

So :
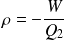
Where
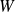 represents the total work received by the fluid during the cycle.
represents the total work received by the fluid during the cycle.
According to the first law :
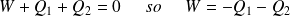
Whence :
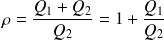
Entropy balance for the fluid in one cycle is :

With :
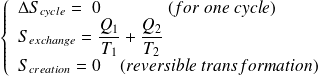
Whence :

We deduce that :
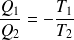
And :

This yield is always less than
 ; for example, with :
; for example, with :
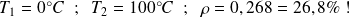
Note that this performance does not depend on the nature of the fluid which undergoes the cycle (perfect gas, real gas, water, ...), but only the temperatures of hot and cold sources.
Engine of irreversible Carnot :
The Carnot cycle is now irreversible (for example, heat transfer is no longer reversibly in contact with two heat sources).
We will show that the efficiency of this irreversible cycle is lower than the reverse cycle, operating between the same two sources.
Entropy balance for the fluid in one cycle is :

With :
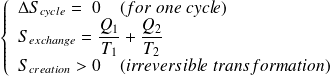
Whence :
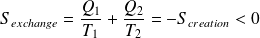
And :
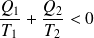
Is the inequality of Clausius.
The efficiency is always defined by :
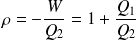
But Clausius gives :

Where : (Carnot's theorem)

Carnot's refrigerating machines :
The Carnot cycle is now gone in the other direction (counterclockwise wise).
If we look at the hot source, this refrigerating machine is a heat pump.
If we look at the cold source, the refrigerating machine is a refrigerator (or air conditioner).

Efficiency of a heat engine (reversible case) :
Refrigerator | Heat pump |
Energy supplied :
We want :
Efficiency :
First law :
Whence :
Clausius equality (always valid) :
Whence :
| Energy supplied :
We want :
Efficiency :
First law :
Whence :
Clausius equality (always valid) :
Whence :
|
Numerical applications :
For a refrigerator :

For heat pump :
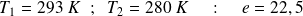
This result shows that
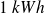 spent to operate the heat pump supplies as much heat as Joule dissipation of
spent to operate the heat pump supplies as much heat as Joule dissipation of
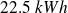 of electrical work in an electric heater !
of electrical work in an electric heater !
In the case of an irreversible operation :