Kinetic theory of ideal gas
Fondamental : The macroscopic model of ideal gas
At the macroscopic scale : a perfect gas is a (real !) gas studied at low pressures (less than a few bars,
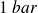 is the normal atmospheric pressure).
is the normal atmospheric pressure).
Gas is characterized by measurable macroscopic parameters on our scale : volume
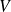 , pressure
, pressure
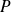 , temperature
, temperature
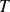 (in Kelvin) or
(in Kelvin) or
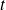 (
(
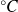 ) and the number of moles
) and the number of moles
 .
.
These parameters are called "state variables".
These variables are defined as "thermodynamic equilibrium" of gas.
Relationship between
 (Kelvin scale) and
(Kelvin scale) and
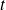 (Celsius scale) :
(Celsius scale) :
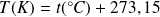
In the IS (the International System), the unit of pressure is the Pascal.
Also used the bar :
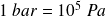
The equation of state of an ideal gas is :
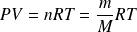
Where
 is the number of moles,
is the number of moles,
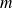 the mass of gas and
the mass of gas and
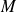 its molecular weight.
its molecular weight.
Fondamental : The microscopic model of ideal gas
Consider a monatomic gas (Ar, He, H, ...) at temperature
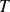 , pressure
, pressure
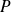 and occupying a volume
and occupying a volume
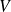 .
.
We note :
 : number of moles
: number of moles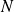 : total number of particles in the volume
: total number of particles in the volume
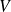
 : density (number of particles per unit volume),
: density (number of particles per unit volume),
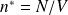
Assumptions of the model :
The particles have a constant movement (thermal agitation).
The particles do not interact with each other but only with the wall during impacts therewith. They are punctual.
The velocities of the particles are distributed in a statistical law called "Maxwell -Boltzmann's distribution law".
The distribution of particle velocities is homogeneous (independent of the point where we place) and isotropic (independent of the chosen direction).
The particle density is the same at all points of the container (homogeneous distribution of the particles).
Fondamental : Calculation of the kinetic pressure
In the following, we choose a simple (simplistic) model of molecular speed distribution law :
The particles (mass
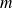 ) all have the same speed (in norm), denoted
) all have the same speed (in norm), denoted
 .
.The particles can move in three dimensions (Ox, Oy and Oz) and therefore six directions.
The kinetic pressure
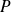 results of incessant impacts of particles against the wall.
results of incessant impacts of particles against the wall.At particle scale, these shocks are elastic (conservations of momentum and kinetic energy).
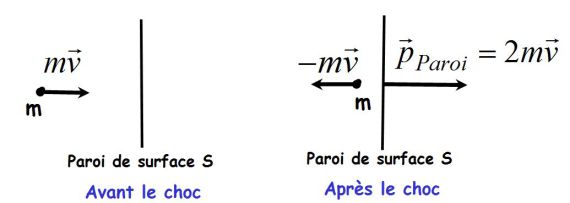
The momentum gained by the wall in an impact is (conservation of momentum) :

During the time interval
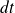 , the force exerted by the wall is :
, the force exerted by the wall is :
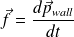
Where
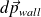 is the amount of movement received by the wall in the impact of particles falling on the wall during
is the amount of movement received by the wall in the impact of particles falling on the wall during
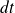 .
.
If
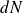 is the number of particles that shock the wall during the time interval
is the number of particles that shock the wall during the time interval
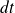 , then :
, then :
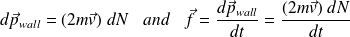
The kinetic pressure
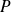 is deduced :
is deduced :
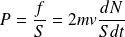
Calculation of
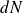 (see figure below) :
(see figure below) :
The particles have a "six chance" to hit the wall :
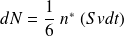
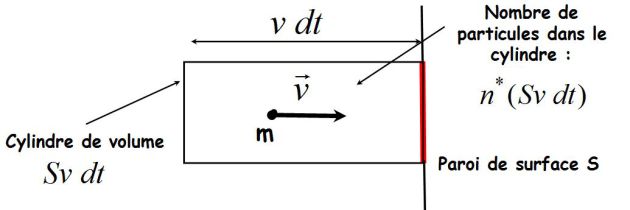
We deduce the kinetic pressure P :
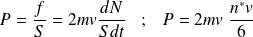
So :
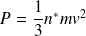
A more realistic calculation, taking into account the Maxwell-Boltzmann distribution led to a similar result, if we replace
 by the quadratic (RMS) speed
by the quadratic (RMS) speed
 :
:
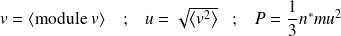
Complément : A video on kinetic pressure
A video on the calculation of the kinetic pressure for a perfect monatomic gas :
Fondamental : Relationship with macroscopic model of perfect gas
Knowing that :

It comes :

This equation is to compare to the macroscopic equation of state of a perfect gaz :
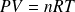
Yet :
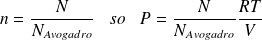
Whence :
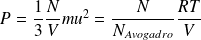
Thus deduced in the expression of the quadratic (RMS = root mean square) velocity
 in function of the absolute temperature
in function of the absolute temperature
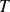 :
:

Where
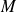 is the molar mass.
is the molar mass.
We note :

the Boltzmann constant (
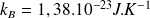 ).
).
Finally :
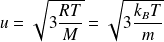
Some numerical applications :
At
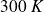 , for helium,
, for helium,
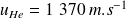 and for Argon,
and for Argon,
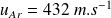 .
.
Fondamental : Kinetic interpretation of the temperature
The average kinetic energy of a particle, denoted
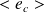 , is expressed in terms of temperature
, is expressed in terms of temperature
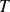 :
:

Temperature is a measure of the thermal motion of the particles.
It is convenient sometimes to express a temperature
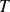 in
in
 ,
,
 or
or
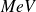 (which are energy units) : the correspondence between
(which are energy units) : the correspondence between
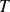 in
in
 and
and
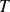 in
in
 is obtained from the previous relationship.
is obtained from the previous relationship.
Thus, if
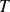 is
is
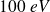 , whereas, in
, whereas, in
 :
:
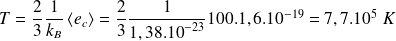
Fondamental : The internal energy of a monoatomic ideal gas
Since we neglect the forces of mutual interaction between particles, the potential energy of mutual interaction (defined in mechanics, in the case of two interacting bodies for instance) is zero.
In the laboratory frame (in which the container containing the gas is assumed immobile), the mechanical energy of the system of
 particles is called "internal energy" and noted
particles is called "internal energy" and noted
 .
.
It is :
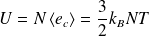
It represents the energy contained within the container, due to the thermal agitation (whence the name of internal energy).
With :
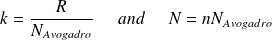
It comes :
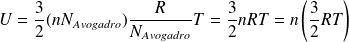
Thus :
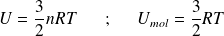
Complément : Case of a perfect diatomic gas
We assume that for a diatomic ideal gas, equation of state is identical to that of a perfect monoatomic gas.
By cons, the molecule capable of oscillate about its mean equilibrium position, the internal energy contains additional terms.
Two additional "degrees of freedom" appear and internal energy is (at normal temperatures that are considered) :

Complément : The Van der Waals' equation of state
Van der Waals (Dutch physicist, 1837-1923) proposed an equation of state which takes into account both the volume of the particles and the existence of the average of attractive intermolecular forces (at long distance).
This is a phenomenological equation of state (based on experiment), which is written (for one mole of gas) :
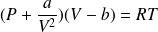
a and b are constants characteristic of the gas.
For
 moles, we have :
moles, we have :

We find, if we neglect the corrective terms, the ideal gas equation,
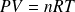 .
.
The realistic volume accessible to molecules is of the order of
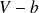 , where
, where
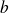 (called molar co-volume) is the volume occupying molecules if they were stacked (pressed) against each other :
(called molar co-volume) is the volume occupying molecules if they were stacked (pressed) against each other :
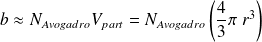
where
 is the radius of a supposed gas spherical particle (of the order of
is the radius of a supposed gas spherical particle (of the order of
 ).
).