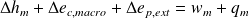First "industrial" law of thermodynamics
Fondamental : Internal energy and enthalpy balances (example of the jet engine)
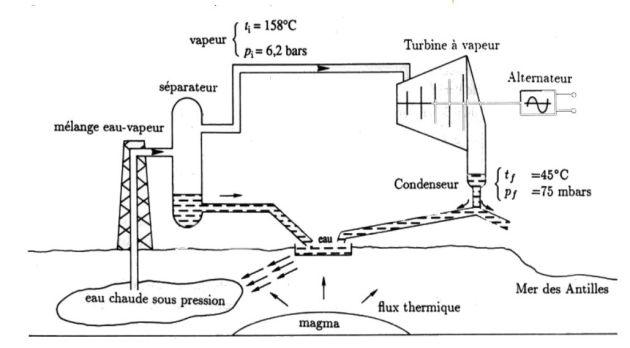
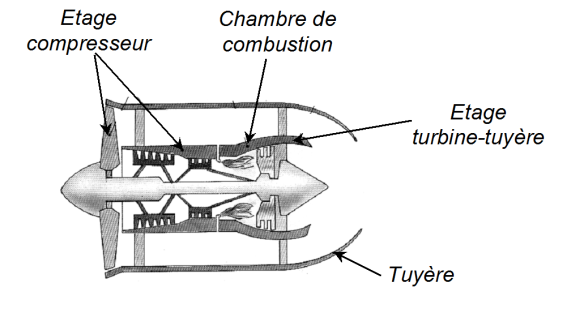
In a jet engine, a gas (likened to the air assumed perfect) runs through a cycle which will be considered as being reversible.
It enters the reactor at the pressure
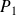 and temperature
and temperature
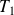 (state
(state
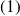 ).
).
It is then compressed adiabatically to the pressure
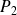 and the temperature is then
and the temperature is then
 (state
(state
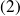 ).
).
It then enters a combustion chamber where its temperature changes from
 to
to
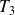 , pressure remains equal to
, pressure remains equal to
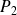 (the output of the combustion chamber is represented by state
(the output of the combustion chamber is represented by state
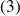 ).
).
The gas then undergoes adiabatic expansion in a turbine
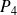 and
and
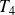 (state
(state
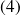 ).
).
This expansion is such that the power supplied to the turbine compensates exactly that which consumes the compressor between the states
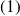 and
and
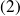 .
.
Finally, the gas expands in an adiabatic nozzle without moving parts and up to
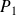 and
and
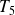 (state
(state
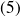 ).
).
The gas is released with the speed
 (propelling) the outside atmosphere where it cools down to the constant pressure
(propelling) the outside atmosphere where it cools down to the constant pressure
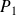 from
from
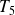 to
to
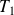 .
.
It is considered that the gas velocity is negligible everywhere except at the output of the nozzle.
Numerical data :
 ,
,
 ,
,
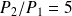 .
.
The gas temperature at the input of the turbine is
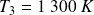 .
.
The air is considered a diatomic gas of molar mass
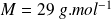 .
.
The requested numerical applications are related to the mass unit (here,
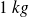 ) and corresponding extensive quantities will be denoted by lowercase letters (
) and corresponding extensive quantities will be denoted by lowercase letters (
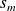 for entropy,
for entropy,
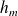 for enthalpy,
for enthalpy,
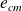 for macroscopic kinetic energy ,...).
for macroscopic kinetic energy ,...).
Fondamental : Compressor study
The unit of air mass that enters the compressor receives only a mechanical work denoted
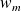 (with
(with
 ).
).
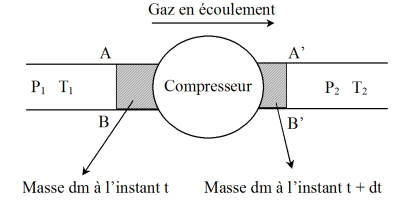
It is considered at the moment
 the closed system consisting of gas included in the compressor and the mass
the closed system consisting of gas included in the compressor and the mass
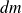 of gas (in the state
of gas (in the state
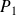 and
and
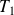 ) that goes back during the time interval
) that goes back during the time interval
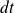 , in the compressor.
, in the compressor.
At the moment
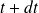 , this system consists of the same amount of gas within the compressor and the same mass
, this system consists of the same amount of gas within the compressor and the same mass
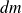 of gas that came out, now being under the conditions
of gas that came out, now being under the conditions
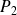 and
and
 .
.
The first law applied to this system (neglecting the macroscopic kinetic energy) is written :
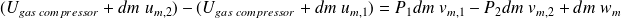
With :
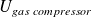 , the internal energy of constantly gas contained in the compressor ; it is constant at steady state.
, the internal energy of constantly gas contained in the compressor ; it is constant at steady state.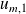 and
and
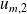 respectively designate the mass internal energies and
respectively designate the mass internal energies and
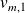 and
and
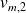 volumes mass of the air in the states
volumes mass of the air in the states
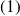 and
and
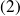 .
.The quantity
 represents the work of external pressure forces in the system at the input and output of the machine (also called a work transfer).
represents the work of external pressure forces in the system at the input and output of the machine (also called a work transfer).Finally, the heat transfer received by the system is zero, since, on the one hand, the compressor is heat-insulated and, secondly, there is no transfer of heat by conduction between the mass which enters or comes out of the machine and its immediate environment as temperatures are identical (and equal to
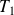 or
or
 ).
).
Noting that :
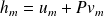
represents the specific enthalpy, it ultimately leads to the following energy balance :

Fondamental : Study of the nozzle
The energy balance in the nozzle is written now, since the macroscopic kinetic energy at output
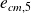 is no longer negligible (and with similar ratings to those of the previous paragraph) :
is no longer negligible (and with similar ratings to those of the previous paragraph) :
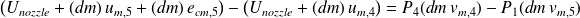
Is :

Attention : "First industrial law"
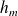 ,
,
 and
and
 designate the specific enthalpy, macroscopic kinetic energy and mass gravitational potential energy of the flowing fluid.
designate the specific enthalpy, macroscopic kinetic energy and mass gravitational potential energy of the flowing fluid.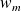 represents the mass work received by the working fluid in the machine (outside of the pressure forces at the input and the ouput of the machine). It is also called "mass useful work".
represents the mass work received by the working fluid in the machine (outside of the pressure forces at the input and the ouput of the machine). It is also called "mass useful work".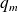 is the specific heat transfer received by the fluid during its passage through the machine.
is the specific heat transfer received by the fluid during its passage through the machine.The "first industrial law" is written for a flowing fluid :